Understanding whether metals have high density is essential in various scientific and industrial applications. Density is a fundamental property that influences how materials behave under different conditions, making it a crucial factor in fields such as engineering, construction, and manufacturing. In this article, we will delve into the concept of density, examine why metals generally have high density, and discuss the implications of this property in real-world scenarios.
Metals are a unique category of elements characterized by their ability to conduct heat and electricity, malleability, ductility, and lustrous appearance. When we talk about the density of metals, we are referring to the mass per unit volume of these materials, which is typically measured in grams per cubic centimeter (g/cm³). This article aims to provide a comprehensive overview of metal density, supported by scientific principles and real-life examples.
As we explore this topic, we will also consider various aspects such as the atomic structure of metals, the role of different elements in determining density, and the practical applications of high-density metals. By the end of this article, you will have a well-rounded understanding of why metals exhibit high density and its significance in both nature and industry.
Table of Contents
- What is Density?
- Density of Metals
- Atomic Structure and Density
- High Density Metals
- Applications of High Density Metals
- Measuring Density
- Factors Affecting Density
- Conclusion
What is Density?
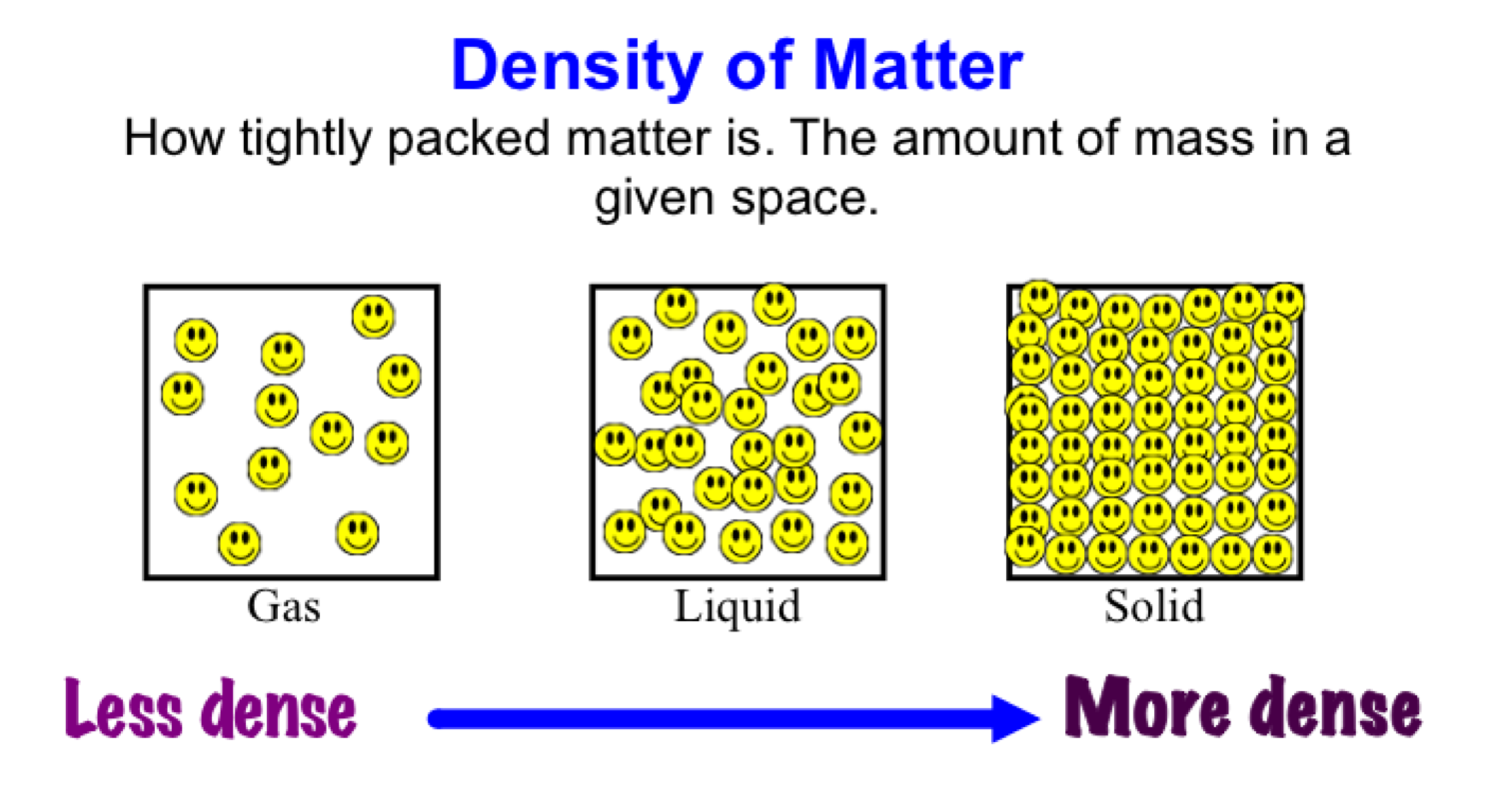
Density is defined as the mass of a substance divided by its volume. It is a key physical property that can provide insights into the composition and behavior of materials. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
In the context of metals, density can vary significantly depending on the type of metal and its atomic structure. For example, lead has a high density of 11.34 g/cm³, while aluminum has a much lower density of 2.70 g/cm³. Understanding density helps in categorizing and utilizing metals for various applications.
Density of Metals
Metals generally have higher densities compared to non-metals. This is largely due to their atomic structure and closely packed arrangements of atoms. Most metals have relatively high atomic masses and small atomic radii, which contribute to their high density. Here are some key points regarding the density of metals:
- Metals such as gold, platinum, and tungsten are known for their high densities.
- The density of metals can be influenced by factors such as temperature and pressure.
- Generally, the denser the metal, the more applications it has in fields requiring strength and durability.
Atomic Structure and Density
The atomic structure of metals plays a pivotal role in determining their density. Metals consist of closely packed atoms arranged in a lattice structure. This close packing allows for a greater mass to occupy a smaller volume, resulting in high density. There are two primary types of metallic bonding that contribute to this structure:
Cubic Close-Packed (CCP) Structure
In the CCP structure, atoms are arranged in a way that maximizes packing efficiency. Metals like copper and aluminum exhibit this structure, contributing to their relatively high density.
Hexagonal Close-Packed (HCP) Structure
Metals such as zinc and magnesium have an HCP structure, which also allows for high packing efficiency and density. The arrangement of atoms in these structures leads to stronger metallic bonds, further enhancing density.
High Density Metals
Some metals are particularly known for their high density, which makes them suitable for specialized applications. The following are examples of high-density metals:
- Gold (Au): With a density of 19.32 g/cm³, gold is not only dense but also highly malleable and ductile.
- Platinum (Pt): Platinum has a density of 21.45 g/cm³ and is valued for its resistance to corrosion.
- Tungsten (W): Tungsten is the densest naturally occurring element, with a density of 19.25 g/cm³, making it ideal for high-stress applications.
Applications of High Density Metals
The high density of certain metals makes them invaluable in various industries. Here are some notable applications:
- Aerospace Industry: High-density metals are used in aircraft components to ensure strength and durability without adding excessive weight.
- Medical Field: Gold and platinum are often used in dental work and medical devices due to their biocompatibility.
- Military Applications: Dense metals like tungsten are used in armor-piercing ammunition and military vehicles for enhanced protection.
Measuring Density
Density can be measured using several methods, including:
- Water Displacement Method: Involves measuring the volume of water displaced by a solid object to calculate its density.
- Archimedes' Principle: A method based on buoyancy that can be applied to find the density of irregularly shaped objects.
- Mass and Volume Measurement: Directly measuring the mass and volume of a sample to calculate density.
Factors Affecting Density
Several factors can influence the density of metals, including:
- Temperature: As temperature increases, metals can expand, leading to a decrease in density.
- Alloy Composition: The addition of other elements to a metal can alter its density significantly. For example, adding carbon to iron creates steel, which has different density characteristics.
- Impurities: The presence of impurities can also affect the density of metals, potentially increasing or decreasing it depending on the nature of the impurities.
Conclusion
In conclusion, metals are characterized by their high density, which is primarily influenced by their atomic structure and arrangement. Understanding the density of metals is crucial for their application in various fields, from aerospace to medicine. High-density metals like gold, platinum, and tungsten play significant roles in industries requiring strength and durability. By measuring density and considering factors that affect it, we can better utilize metals in our everyday lives.
We invite you to share your thoughts on this topic or ask any questions in the comments below. If you found this article informative, consider sharing it with others or exploring our other articles for more insights!
Thank you for reading, and we hope to see you again soon for more fascinating discussions on materials science and engineering!